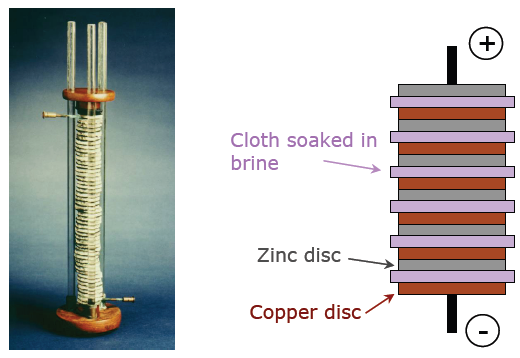
The lead acid battery was the first rechargeable battery, first created by French physicist Gaston Plante in 1859. Despite having been outclassed by lithium ion in terms of energy density, still remains widely used for its low cost and ability to supply large currents. Lead-acid batteries are the second most manufactured battery type after lithium ion.
Chemistry
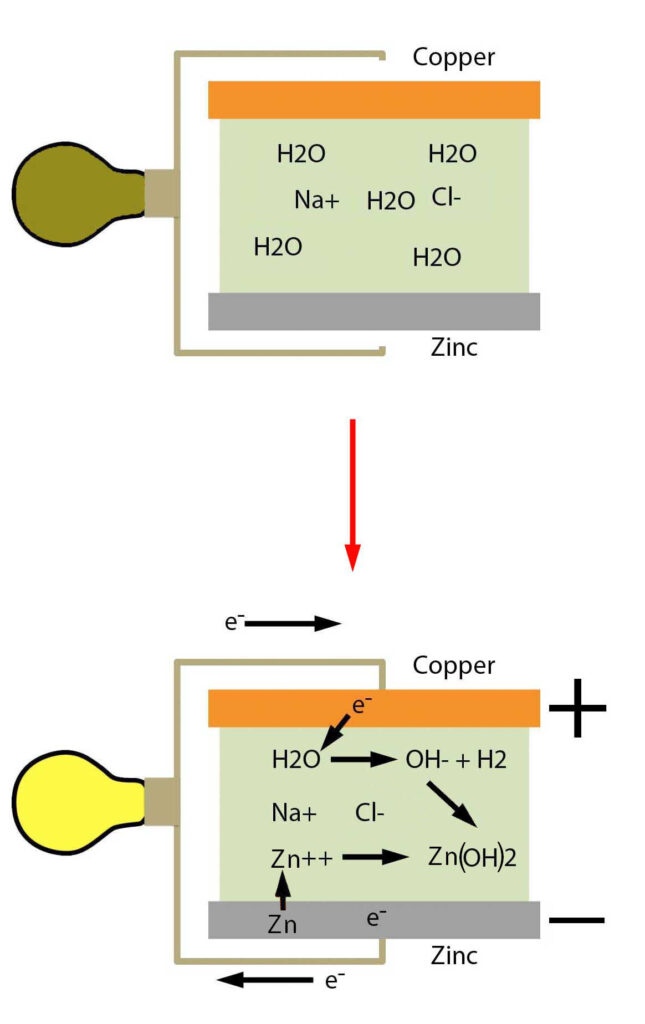
A fully charged lead acid battery consists of a cathode made of lead dioxide, an anode of lead metal, and sulfuric acid as the electrolyte. The lead metal plate being the anode we know that the lead must be undergoing oxidation.
![]()
The Pb2+ then combines with HSO4– in solution to form a solid precipitate of PbSO4 around the lead anode. This releases the hydrogen attached to the sulfate which would make the full half reaction occurring at the anode:
![]()
At the lead dioxide cathode we know the lead dioxide must be getting reduced. The lead atom in lead dioxide has an oxidation number of +4 due to its two oxygens which means that it can be reduced by also becoming Pb2+. To facilitate this, the hydrogen ions produced by the reaction in the anode are consumed in this reaction to remove the two oxygens as two waters.
![]()
This would make the overall reaction:
![]()
As you can see, in this reaction the acid is consumed and converted to water which means, in its discharged state, a lead acid battery would have both of its electrodes coated in lead sulfate and the sulfuric acid would have been almost entirely consumed.
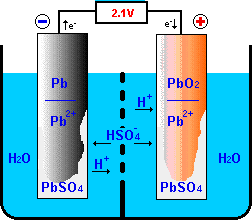
When the battery is charged the reaction is reversed and the two electrodes return to their original state. Of course, this process is not perfect which leads the battery losing some capacity every charge cycle. One mechanism by which this occurs is through sulfation in which the lead sulfate produced by the reaction forms crystals in such a way that renders the lead sulfate unable to participate in the reaction again. In some cases, sulfation can be reversed, but not always.
Construction
While early lead acid batteries were as simple as two plates submerged in acid, modern lead acid batteries use a variety of manufacturing techniques to maximize power and minimize electrode corrosion.
Electrodes
One feature of every modern lead acid battery cell is that each electrode being a single plate, such as one you would use in a simple lab set up, each electrode consists of several attached plates. This increases the surface area of the battery which allows more mols of the reaction to occur simultaneously which increases the flow of electrons across the battery. Note that this does not increase the voltage of the cell, the voltage will always be more or less the same despite configuration. But it does allow more electrons to travel at a given time. The increase in surface area could thus be thought of as decreasing the internal resistance of the battery which allows for a higher maximum current. The standard potential of one cell is 2.05V. Thus, the average 12V lead acid battery would be made up of 6 of these cells.
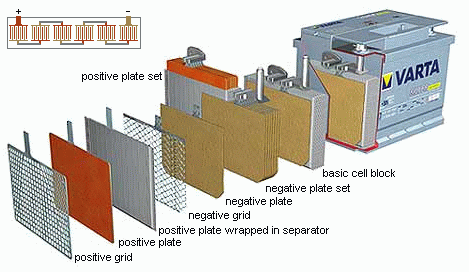
In the above image you can see how each cell consists of interlocking plates that are kept apart by a separator that allows ions to pass through. Note that each plate is only attached to plates of the same electrode.
Anode
The anode needs to be made of lead, however, pure lead is too soft to maintain its shape. Therefore, lead alloys are used to strengthen the plates and improve the electrical properties of the electrodes. Some added metals include tin, selenium, calcium, and antimony. Each metal contributes differently to the performance of the battery and thus batteries with different intended purposes will use different alloys. For example,
Cathode
The cathode is made up of plates coated with a lead oxide paste. Modern lead acid batteries include various additives to the paste to improve performance. One example is barium sulfate which acts as a seed crystal for the formation of lead sulfate crystals. When the lead sulfate forms in these crystals, it is easier for the reverse reaction to occur and convert the lead sulfate back to lead oxide. Other additives can be introduced to the electrolyte to boost performance.
Separators
Another innovation in the lead acid battery was improvement of the separator. Many lead-acid batteries now use absorbent glass mat separators(AGMs). These are mats made of glass fibers which soak up the electrolyte. This is advantageous compared to a flooded lead acid battery because if the battery is punctured, no acid will leak out. Also, since the electrolyte is confined to the glass mat, the battery can now experience more turbulence without potentially damaging the battery.
Uses
Most of the world’s lead-acid batteries are used to start the engines and power the electrical systems of automobiles. Most of the stored charge of the lead acid battery isn’t directly used as, once the engine has started, the vehicle’s alternator converts some of the engines power into electricity which powers the vehicle’s electrical systems and charges the battery.
Lead-acid batteries can also be used to power small, fully electric vehicles such as golf carts, electric scooters, electric bikes, and electric wheelchairs.
Lead-acid batteries are also used to store backup power for telecommunication systems.
References/Further Reading
Battery University: How does the lead-acid battery work?
Lead Acid Battery Chart
Battery Charging and Maintenance (images)